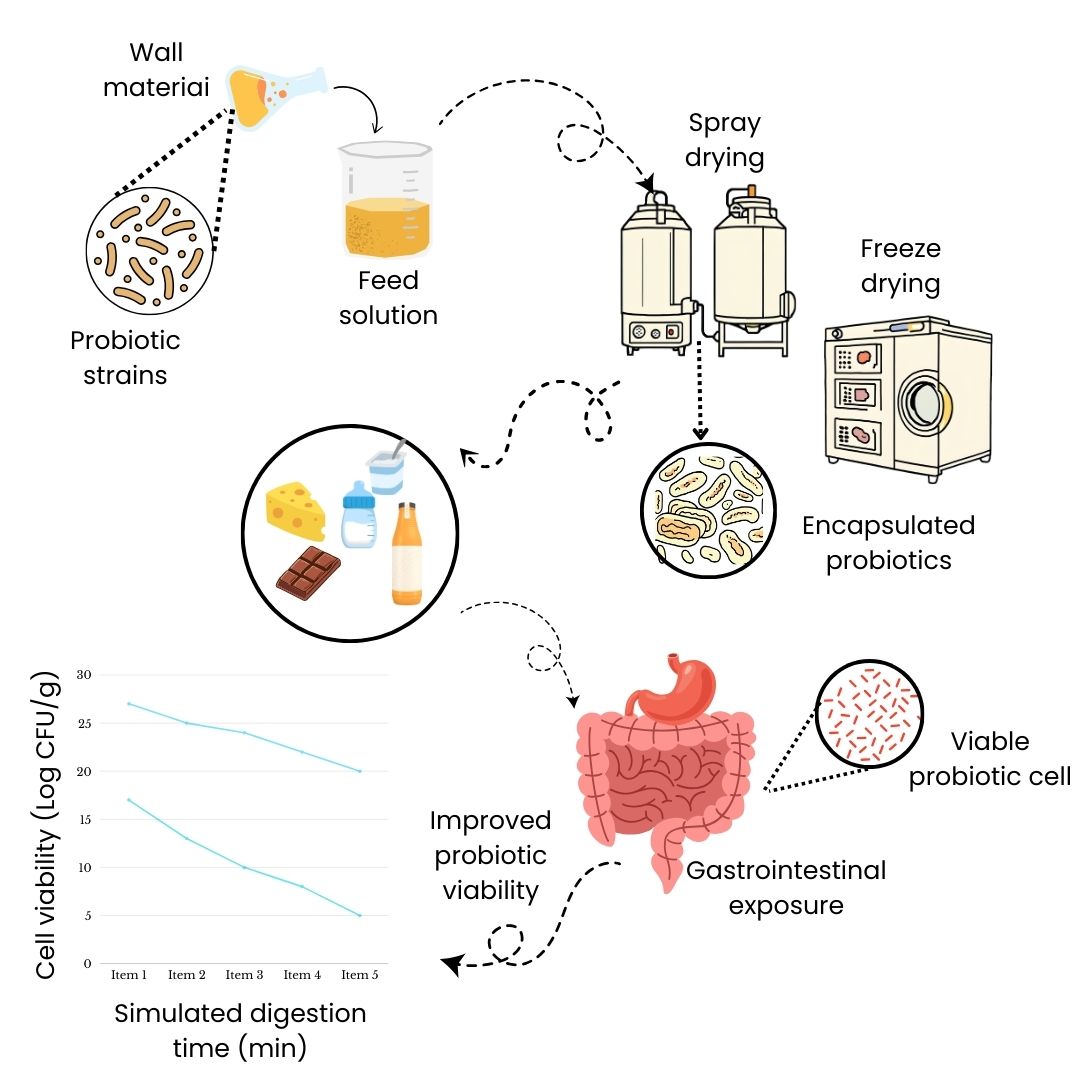
2. Freeze Drying
Description: Freeze drying removes moisture from probiotics by sublimation under low temperature and pressure, resulting in a porous powder.
Encapsulate Structure: Irregularly shaped particles larger than 1 mm, with broad size distribution.
Critical Parameters:
- Condenser Temperature: Must be lower than the product temperature to facilitate sublimation.
- Vacuum Pressure: Optimal pressures range from 0.1–0.5 Torr.