Implications for Manufacturers
Opportunities for Innovation
Manufacturers in the nutraceutical and functional food industries can leverage these findings to:
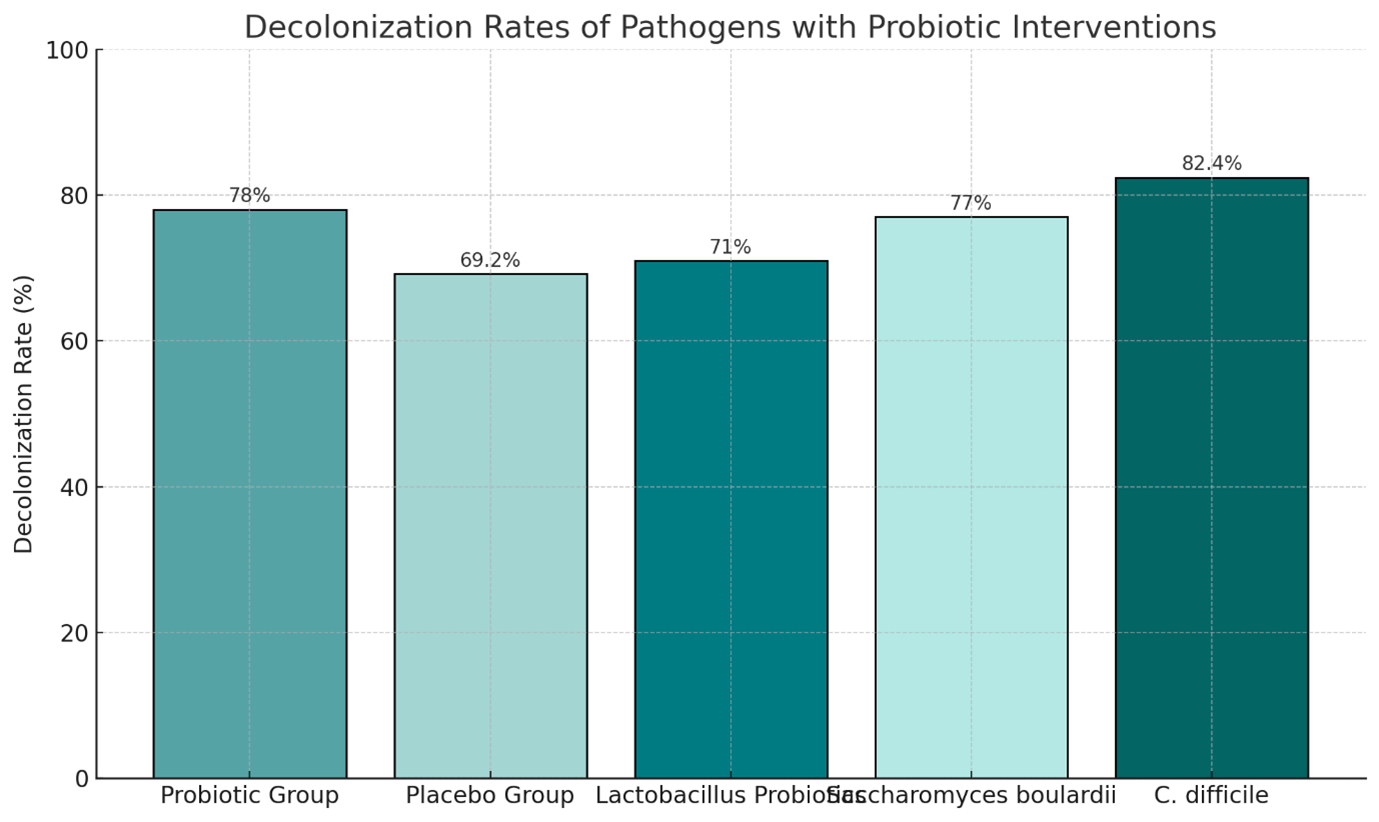
- Develop targeted probiotic formulations using strains like Lactobacillus and Saccharomyces boulardii for gut health and pathogen decolonization.
- Create synergistic prebiotic-probiotic blends to enhance gut microbial diversity.