LATEST REGULATION: FSSAI DRAFT NO NORMS
Regulations
Nutraceuticals Regulations FSSAI
- Food
- Regulations
- Nutraceutical
- cosmeceutical
- Pet Food Product
- Herbal Product
- Agriculture Product
Nutraceuticals Regulations FSSAI
Table of Content
- 1. Permissible/permitted overages in food formulations
- 2. Overages needed in functional or fortified food products
- 3. Standard permissible overages as per regulation
- 4. Permissible/permitted limit in the food formulation
- 5. Amino acids and nucleotides
- 6. Commonly permitted amino acids and their derivatives
- 7. Nucleotides are permitted in functional food and beverage
- 8. Scientifically justifying higher overages
- 9. FRL support the formulation of amino-acid- and nucleotide
1. What are the permissible/permitted overages in food formulations?
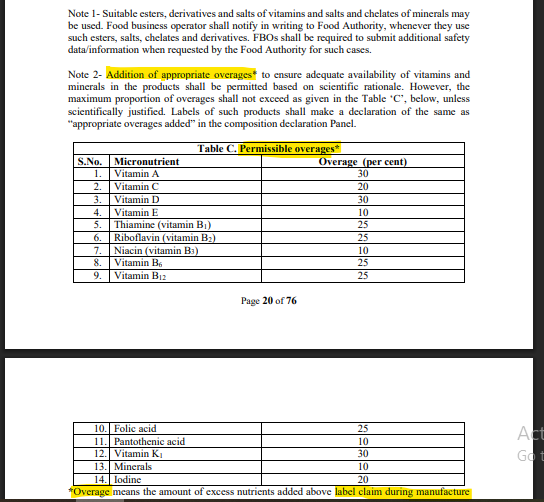
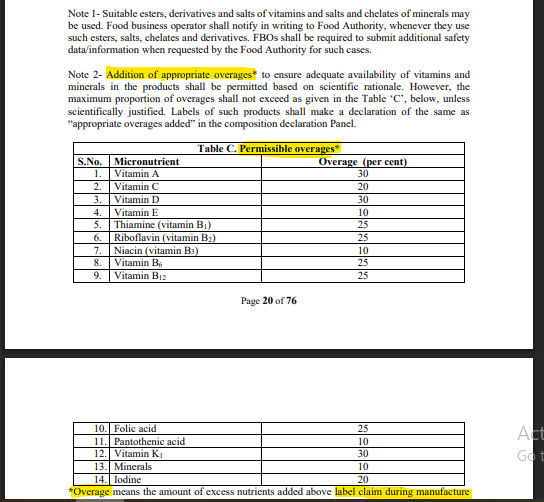
Permissible overages are those amounts of micronutrients in the manufacture of the product to allow potential losses through processing and storage. This ensures that the levels are still retained at the time of consumption by the manufacture of the product. FSSAI and other regulatory authorities permit specific percentages for each nutrient (Vitamin A: 30%, Vitamin C: 20%, etc.).
[Refer Annexure 2, Schedule 1, Page 20 of 76, Note 2, Table Under Covered, Section (C) Permissible overages, F. No. Std/SP-05/T(Nutraceuticals-2022) [E-5184], 29th March 2022].
2. Why are the overages needed in functional or fortified food products?
The overages would guarantee the product’s nutritional integrity until the end of its shelf life. Overages offset natural losses incurred over time and losses possible due to depreciation from manufacturing, storage, and transport to keep nutrient values claimed on the label.
[Refer Annexure 2, Schedule 1, Page 20 of 76, Note 2, Table Under Covered, Section (C) Permissible overages, F. No. Std/SP-05/T(Nutraceuticals-2022) [E-5184], 29th March 2022].
3. What are the standard permissible overages as per regulation?
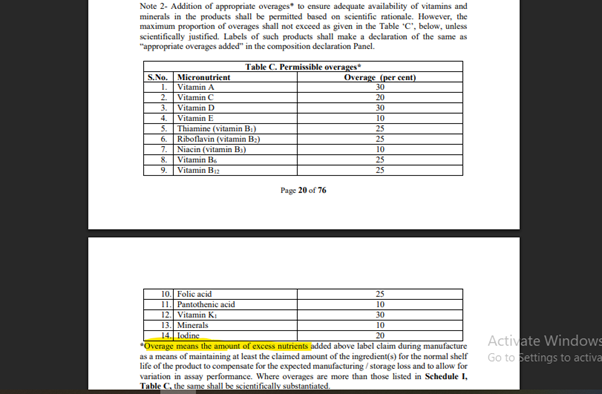
Given Below is a reference table of permissible/permitted overages as per the regulation:
| S.No. | Micronutrient | Permissible Overage (%) |
| 1 | Vitamin A | 30% |
| 2 | Vitamin C | 20% |
| 3 | Vitamin D | 30% |
| 4 | Vitamin E | 10% |
| 5 | Thiamine (B1) | 25% |
| 6 | Riboflavin (B2) | 25% |
| 7 | Niacin (B3) | 10% |
| 8 | Vitamin B6 | 25% |
| 9 | Vitamin B12 | 25% |
| 10 | Folic Acid | 25% |
| 11 | Pantothenic Acid | 10% |
| 12 | Vitamin K1 | 30% |
| 13 | Minerals | 10% |
| 14 | Iodine | 20% |
[Refer Annexure 2, Schedule 1, Page 20 of 76, Note 2, Table Under Covered, Section (C) Permissible overages, F. No. Std/SP-05/T(Nutraceuticals-2022) [E-5184], 29th March 2022].
4. What if the overages exceed the permissible/permitted limit in the food formulation?
A food regulatory body could be requested to provide scientific justification and stability data for any formulation that falls under the category of excess average limit. This ensures product safety and safeguards consumers against unforeseen dangers posed by the products they utilize.
[Refer Annexure 2, Schedule 1, Page 20 of 76, Note 2, Table Under Covered, Section (C) Permissible overages, F. No. Std/SP-05/T(Nutraceuticals-2022) [E-5184], 29th March 2022].
5. Can amino acids and nucleotides be added to functional foods and beverages?
YES. Besides having access to a vast variety of amino acids and nucleotides in formulations for therapeutic nutrition, sports drinks, infant nutrition, and medical foods, these ingredients play important roles in metabolism, immunity, muscle recovery, and cognition.
[Refer Annexure 2, Schedule 1, Page 21 of 76, Note 2, Under List of amino acids, other nutrients and Nucleotides Section (A), (B), List of amino acids, other nutrients and Nucleotides, F. No. Std/SP-05/T(Nutraceuticals-2022) [E-5184], 29th March 2022].
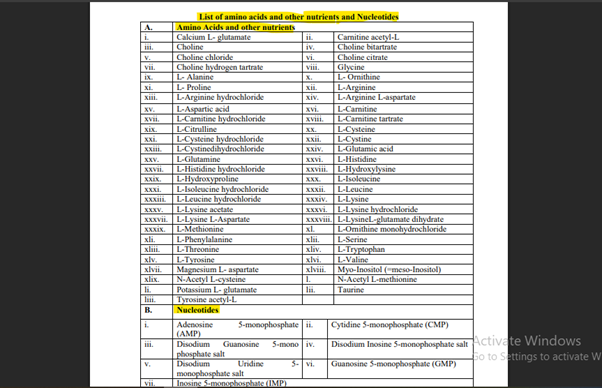
The outline for amino acids which are permitted:
- Essential amino acids: L-Tryptophan, L-Isoleucine, L-Lysine, L-Leucine, L-Methionine, L-Valine, L-Threonine, L-Phenylalanine.
- Conditionally essential: L-Arginine, L-Cysteine, L-Glutamine, L-Histidine
- Others:L-Carnitine, Glycine, Taurine, N-Acetyl ones, Choline (multiple forms)
Note: These above mentioned might appear as free amino acids, salts (hydrochloride, sodium, potassium), or hydrated forms with prior reference to the authority.
[Refer Annexure 2, Schedule 1, Page 21 of 76, Note 2, Under List of amino acids, other nutrients and Nucleotides Section (A), (B), List of amino acids, other nutrients and Nucleotides, F. No. Std/SP-05/T(Nutraceuticals-2022) [E-5184], 29th March 2022].


The following nucleotides are allowed:
-
- Adenosine 5-monophosphate (AMP)
- Cytidine 5-monophosphate (CMP)
- Guanosine 5-monophosphate (GMP)
- Inosine 5-monophosphate (IMP)
- Uridine 5-monophosphate (UMP)
- Disodium salts for improved solubility
These are specifically beneficial for gut health and for immune support in the fields of infant nutrition and clinical nutrition.
[Refer Annexure 2, Schedule 1, Page 21 of 76, Note 2, Under List of amino acids, other nutrients and Nucleotides Section (A), (B), List of amino acids, other nutrients and Nucleotides, F. No. Std/SP-05/T(Nutraceuticals-2022) [E-5184], 29th March 2022].

Yes, Food Research Lab can even assist the customer in conducting trail stability studies into scientific dossiers and submission for regulatory authorities at higher overages or new ingredient introduction.
9. How does FRL support the formulation of amino-acid- and nucleotide-rich products?
The role of FRL sits on an end-to-end services basis:
- Ingredient sourcing and authentication
- Assistance toward Regulatory Compliance (FSSAI, FDA, EFSA)
- Assistance in stability and assay of products
- Clinical and scientific substantiation
- Validation of label claims and technical documents

Let’s create something Innovative and Delicious together
Food Research Lab strives for excellence in new Food, Beverage and Nutraceutical Product Research and Development by offering cutting edge scientific analysis and expertise.




