In recent years, the nutraceutical, nutrition, and functional food industry has drawn attention to its products, which are great sources of vitamins, minerals, fibre, prebiotics, probiotics, symbiotic, postbiotics, proteins, and low glycemic carbs and may offer health benefits beyond basic diet [3]. However, this growth increases the need for product safety, quality, and regulatory compliance. To achieve this goal, the manufacturers should implement the Certificate of Analysis (CoA) as an essential process.
Why You Need a Certificate of Analysis (COA)?
Interesting News, August 12, 2024.

Why You Need a Certificate of Analysis (COA) in the Nutraceutical, Nutrition and Functional Food Industry - Ensuring Quality, Safety and Adherence to Product Standards

Start your beverage
brand today

To review your food with Professional
staying one Step ahead
Introduction
In recent years, the nutraceutical, nutrition, and functional food industry has drawn attention to its products, which are great sources of vitamins, minerals, fibre, prebiotics, probiotics, symbiotic, postbiotics, proteins, and low glycemic carbs and may offer health benefits beyond basic diet [3]. However, this growth increases the need for product safety, quality, and regulatory compliance. To achieve this goal, the manufacturers should implement the Certificate of Analysis (CoA) as an essential process.
Definition and Significance of a Certificate of Analysis (CoA)
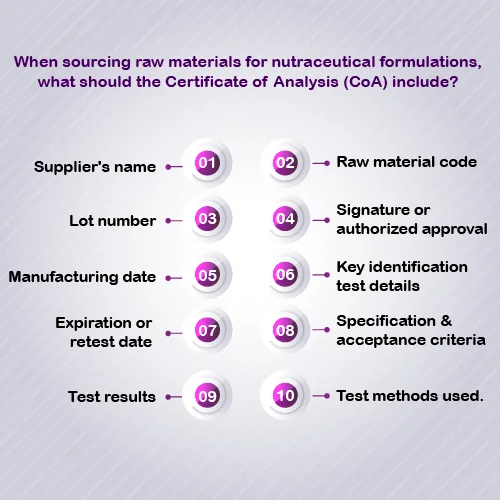
CoA is a document which describes the necessary characteristics, such as appearance, purity, solubility, and water content, as well as the analytical standards, such as name, CAS number, molecular formula, and molecular weight [1]. In the context of the nutraceutical, nutrition, and functional food industry, CoA documents act as an identification document or serve as a basis for product comparison. As it provides information on the nutritional value, storage conditions, and other properties of the product, such as purity [2]. It acts as an essential document that demonstrates the safety, quality, and adherence to regulations of the product and guarantees that manufacturers goods comply with industry regulations and customer expectations.
Overview of the Nutraceutical, Nutrition, and Functional Food Industry
The nutraceutical, nutrition, and functional food industry departure from traditional diets, introducing a new dimension to the concept of nourishment. These industries cover a vast array of products, comprising functional foods, herbal goods, vitamins, and minerals, as well as dietary supplements [1]. These products are designed to provide specific health benefits beyond basic nutrition. Given the complex nature of these products and the potential impact on consumer health, rigorous quality and safety measures are paramount.
Identification Test Details
An identification test verifies that the ingredient being purchased is indeed the intended one. It serves as a crucial safeguard for manufacturers to prevent both intentional and unintentional adulteration, while also ensuring that the correct ingredients are used in their products. The method for the identification test must align with the specifications outlined. Test results should detail the lot number, reference standard, and an explanation of why that standard is appropriate. Additionally, the test method protocol, including sample preparation, experimental conditions, and detection techniques, should be clearly documented.
Quality Assurance
- Ensuring raw materials and finished product quality:
A CoA relevant parameter of raw materials including the identity, purity, potency, and other relevant parameters of raw materials. This information is used to confirm the specified standard required for the raw materials. Similarly, CoAs is used to confirm that final products are aligned with quality standard designed for the raw materials.
Botanical ingredients, especially extracts, are often mixed with other components like carriers and excipients. To provide transparency, a composition statement should be available, detailing all subcomponents present in the raw material or confirming that the botanical ingredient makes up 100% of the material. This document is essential for assessing the feasibility of formulating final products and for creating an accurate ingredient list. It may also determine whether additional regulatory documentation is needed. The composition statement should list all subcomponents, including excipients and preservatives, specify the source of any subcomponent if there are regulatory concerns, and provide a quantitative amount or a reasonable range for each. These subcomponents must align with those listed in another key document, the manufacturing flowchart.
A manufacturing flowchart outlines the key steps involved in transforming starting materials into finished raw materials. Understanding the general manufacturing process is crucial as it can help anticipate the chemical composition of the final product, especially with botanically derived complex ingredients, where the process significantly influences the composition. Although some details may be proprietary, a manufacturing flowchart should at least include information on the starting material(s), mixing, extraction methods, solvents used, excipients, drying process, and the final raw material, listed in the order of execution. These flowcharts must be specific to the ingredient, not generic, and should offer detailed insights into critical processing steps such as grinding, extraction, drying, powdering, and packaging. A flowchart lacking such details provides minimal value.
Importance of CoAs in maintaining consistent product quality: The COA used not only for product inspection it serves beyond that. Moreover, it plays an important role in controlling the product consistency by providing comparison data and monitoring the performance of the product. By checking variation in CoA data, manufacturers have the possibilities to detect quality problems accurately and implement required control measures.
Safety Compliance
- Ensuring products are safe for consumer use: according to the food sector, consumer safety is essential, in this context CoAs can be used to identify any pollutants, allergies, or risks in raw materials or final goods. It helps to make the product follow the necessary safety standards.
- Identification and mitigation of potential contaminants: CoAs provide information on the presence of contaminants such as heavy metals, pesticides, or microbial impurities. Manufacturer CoA verification is crucial for ensuring ingredient quality compliance, mitigating errors and risks, and complying with industry regulations.
- Contaminant specifications should be tailored to the specific ingredients involved.
- For extracts, it is recommended to provide documentation showing residual solvents and their levels.
- In some countries, regulations require the reporting of residual solvent levels (i.e., solvents that remain in the raw material after processing) to ensure they are safe for consumption.
- The reported residual solvents should align with those expected from the manufacturing process and must remain consistent across all related documents, including the CoA, specification sheet, and manufacturing flowchart.
- When included in a CoA, residual solvent data must:
- Clearly state the specification limit and the actual test result.
- Use precise test results; “conforms” is not a valid result.
Regulatory Adherence
- Meeting industry-specific regulatory requirements (FSSAI, EFSA, FDA): Without necessary compliance, the nutraceutical, nutrition, and functional food industry are difficult to run. The documentation provided by the COAs are adhered to the regulations set forth by the Food Safety and Standards Authority of India (FSSAI), the European Food Safety Authority (EFSA), and the Food and Drug Administration (FDA).
- How CoAs support compliance with international standards: The information on product composition and testing results is provided by CoA which can aid other countries in aligning with the international standards for food safety and quality.
Transparency and Traceability
- Enhancing transparency in the supply chain: CoAs provide clear information on origin and characteristics of raw products which can contribute to transparency in supply chain. This transparency is considered as the key tool to build trust with consumers and regulatory bodies.
- Traceability of product ingredients and origins: The manufacturers can easily trace the problem’s origin and find defective products using the CoAs and this process can be used in crisis management.
Building Consumer Trust
- The impact of CoAs on consumer confidence and brand reputation: COAs can be used as a tool to develop trust by reducing the compliance risk and it is used to remove non-conformance issues which can affect brand. Then by ensuring transparency, this COA can increase the confidence in the customer.
- Case studies or examples of how CoAs have helped companies gain trust: CoAs, issued by third-party laboratories, are crucial for ensuring product quality and safety in various industries like dietary supplements, pharmaceuticals, food, and cosmetics.
Facilitating Market Access
- CoAs as a requirement for entering new markets: Imports process requires accurate CoAs to reduce the hurdles in the regulatory process and helps to streamline the market entry process easily.
- How COAs can simplify the process of regulatory approvals: CoA simplifies the regulatory process by providing detailed product information which can speed up the approval process and decrease the time and resources required for this process.
Supporting Product Claims
- Validation of product health and nutritional claims through scientific data: CoA provides nutritional information such as supplier information, materials identity, signature data and test results. It is for consumers, including product value, storage conditions, and purity. CoA also includes information such as nutritional and health claims that are essential for substantiating marketing claims and complying with industry regulations [4].
- The importance of CoA s in marketing and labeling: The information required for labelling also clearly mentioned in CoA s which are essential for substantiating claims using scientific
Risk Management
- Reducing the risk of recalls and product failures: A CoA is very useful in reducing the risk of recalls by ensuring quality meets the required specifications without contamination and putting them in rigorous testing.
- The role of CoA s in proactive risk management strategies: The CoA also acts as a powerful tool in reducing the risk associated with manufacturing. It helps the manufacturers easily detect the possible issues and act on time.
It is not only manufacturers who need to maintain CoA as per the GMP compliance, its private label distributors must have a clear understanding of the manufacturing processes to determine whether a packaged and labeled product meets its established specifications and to decide on product approval and release for distribution. A common way to meet this requirement is by obtaining certificates of analysis (CoAs) from the manufacturer. However, regulations now impose additional requirements for CoAs. In the past, many companies accepted CoAs without further scrutiny, but the FDA, FSSAI, EU and other regulatory agencies now mandates that companies first qualify the supplier or manufacturer by verifying the reliability of their CoAs. Companies must also document how the supplier or manufacturer was qualified and periodically re-confirm the accuracy of the CoAs provided.
Conclusion
It is observed that a Certificate of Analysis is used as the critical tool for ensuring the security, conformance and efficacy of products in the nutraceutical, nutrition, and functional food industries. This CoA is mainly useful in protecting consumer health and enhancing brand trust. Further, it is useful in many areas such as product characteristics confirmation, identifying the risk and supporting legal compliance. Therefore, from this blog it is confirmed that the companies require CoA program to work in a fast pace.
By food research lab contract research and development and manufacturing organization, businesses can significantly enhance operational efficiency and reduce costs. Providing raw material outsourcing services can notably increase business operation efficiency and decrease the expenses associated with raw material procurement.

Let’s create something Innovative and Delicious together
Food Research Lab strives for excellence in new Food, Beverage and Nutraceutical Product Research and Development by offering cutting edge scientific analysis and expertise.