World Health Organization (WHO) reported that more than 80% of the global population relies on traditional medicine for their primary healthcare needs. A sum of 20,000 plants has been identified in 91 countries that contain medicinal properties. Natural compounds obtained from plants contain a wide range of functionalities and properties that can be used to treat chronic as well as infectious diseases. These bioactive compounds are found in very small amounts in plants and certain food product development and can be used to improve good health. Lycopene, resveratrol, lignan and tannins are common bioactive compounds (1). These pure compounds or standardized extracts are reported to have many beneficial activities such as antimicrobial, anticancer, analgesic and wound healing activity. The use of these bioactive compounds in the pharmaceutical, food product industry signifies the need to have a standard method to extract them from the plants. To utilize the biologically active compounds from the plant, the compounds need to be extracted, screened, isolated and characterized. Then the bioactive compound could be evaluated toxicologically and clinically for any nutraceutical supplement development.
General Extraction protocals of bioactive compounds from plants
Introduction
World Health Organization (WHO) reported that more than 80% of the global population relies on traditional medicine for their primary healthcare needs. A sum of 20,000 plants has been identified in 91 countries that contain medicinal properties. Natural compounds obtained from plants contain a wide range of functionalities and properties that can be used to treat chronic as well as infectious diseases. These bioactive compounds are found in very small amounts in plants and certain food product development and can be used to improve good health. Lycopene, resveratrol, lignan and tannins are common bioactive compounds (1). These pure compounds or standardized extracts are reported to have many beneficial activities such as antimicrobial, anticancer, analgesic and wound healing activity. The use of these bioactive compounds in the pharmaceutical, food product industry signifies the need to have a standard method to extract them from the plants. To utilize the biologically active compounds from the plant, the compounds need to be extracted, screened, isolated and characterized. Then the bioactive compound could be evaluated toxicologically and clinically for any nutraceutical supplement development.
Objectives of Plant Extraction
- Firstly, extraction of bioactive compounds from plants
- Secondly, selectivity if the analytical methods needs to be increased
- Thirdly, sensitivity of the bioassay is increased by increasing the concenteration of targeted compounds
- Fourthly, detection and Separation of bioactive compounds into a suitable compound
- Finally, strong and Robust method should be reproducible with sample matrix variations.
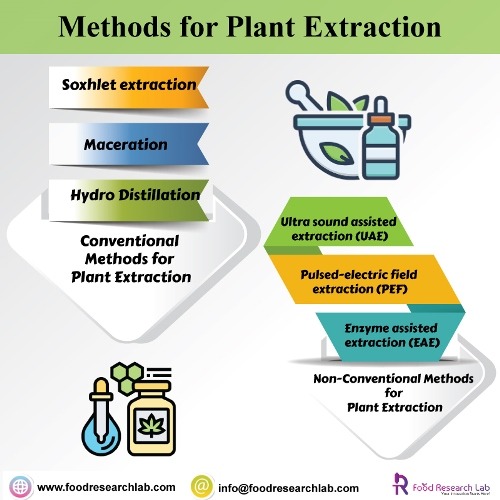
Conventional Methods for Plant Extraction
Soxhlet extraction
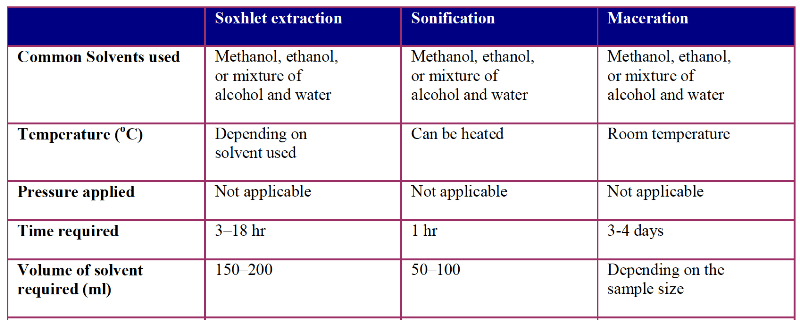
Soxhlet extraction was first used for lipids, however, it not limited any more. A dry sample is placed in a thimble, which is further placed in a distillation flask containing the solvent of interest. Siphon unloads the solution back in the distillation flask. The process is repeated until the final compound of interest is extracted
Maceration
Maceration is commonly used in homemade tonics. It is popular and used to obtain essential oil and bioactive compounds. The plant material is ground into smaller particles and mixed with a solvent. Then the menstruum (solvent) is added to a closed vessel and the liquid is strained to recover the solid residues. The strained liquid is separated from impurities by filtration.
Maceration is an extractive technique that is conducted at room temperature. It consists of immersing a plant in a liquid (water, oil, alcohol, etc.) inside an airtight container, for a variable time based on the plant material and liquid used
Hydro Distillation
Hydro distillation is commonly used for essential oils from plants, which can be done via water, steam or direct steam distillation. In this method, the plant material is boiled in water and direct steam is injected. Indirect cooling condenses the vapour and the separator separates the oil and bioactive compounds from the water. This method is not used for thermolabile compounds.
Table 1 Experimental conditions for various methods of extraction for plants materials. Table adapted from
Non-Conventional Methods for Plant Extraction
There are quite a few modern nonconventional methods which are preferred compared to the conventional methods. Some of the key modern methods are supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), Microwave assisted extraction (MAE), Enzyme assisted extraction (EAE), pulsed electric field extraction (PEF) and Ultra sound assisted extraction (UAE).
Ultra sound assisted extraction (UAE)
Ultrasound passes through a medium by creating cavitation, i.e. compression and expansion. Liquid materials can be exploited using this cavitation effect. UAE facilitates the leaching of organic and inorganic compounds from the plant matrix, by intensifying the mass transfer (4). Ultrasound extraction is found to be suitable for bioactive compounds from herbal plants. It is effective for achieving efficient mixing, faster energy transfer, reduced thermal gradients and selective extraction.
Pulsed-electric field extraction (PEF)
PEF treatment is reported to be useful for improving the pressing, extraction and diffusion process (5). PEF mechanism works on the principle of the destruction of cell membrane structure for enhanced extraction and decreased extraction time. PEF treatment is applied to improve the release of intracellular compounds by increasing the cell membrane permeability (6). Based on the design of the treatment chamber, PEF process could either be operated in batch or continuous process. PEF treatment parameters such as field strength, specific energy, treatment time, temperature and property of the plant matrix are responsible for the effectiveness of the treatment.
PEF treatments at 1kV/cm and 7kJ/kg have been used to extract betanin from beetroots. Moreover, recovery of phytosterols from maize was increased by 33% and isoflavonoids from soybeans were increased by 20% . Application of PEF on grape skins before maceration has been reported to reduce the overall time required with improved stability.
Enzyme assisted extraction (EAE)
This method is widely accepted and used as it is novel, non-toxic and non-inflammable. In some plant matrices, phytochemicals are present in the cell cytoplasm and retained by hydrogen or hydrophobic bonding, which are not accessible by solvent extraction. The addition of specific enzymes such as cellulose, amylase, pectinase as a pretreatment enhances the recovery by breaking the cell wall and hydrolyzing the structural polysaccharides and lipids. Extraction of oils from various seeds can be achieved using enzyme assisted aqueous extraction. The extracted oils were found to contain a higher amount of free fatty acids and phosphorus content compared to the conventional methods. Enzyme composition, concentration, particle size and hydrolysis time are considered as key factors of EAE.
Identification and Characterization
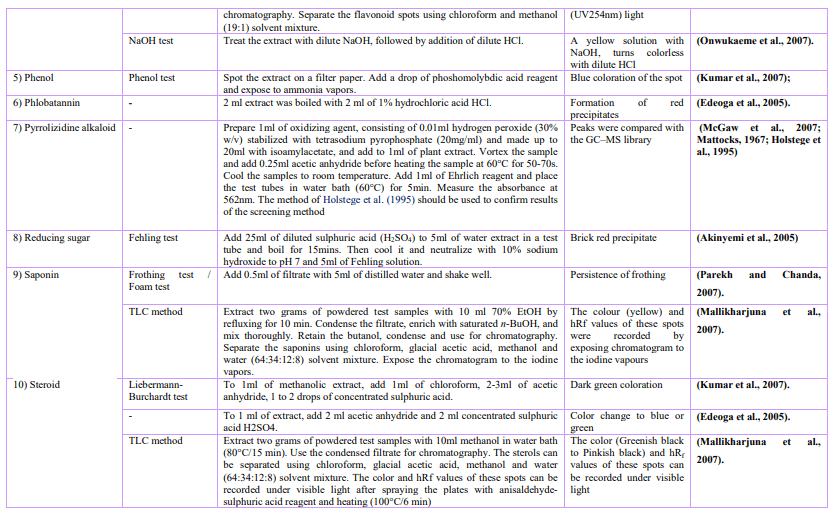
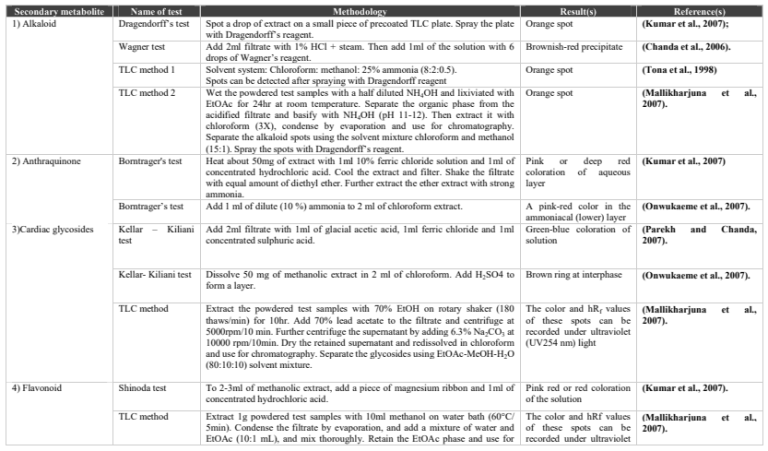
Bioactive compounds could be isolated using chromatography techniques, such as TLC, column, flash, Sephadex and HPLC. The pure compound’s structure is determined and then their biological activity. Non-chromatographic techniques include immunoassay, monoclonal antibodies, phytochemical and Fourier-transform infrared spectroscopy (FTIR).
Conclusion
The increasing demand for plant based bioactive compounds has developed continuous extraction methods. The development of non-conventional extraction procedures and chromatography advancements has resulted in efficient extraction and isolation of bioactive compounds. Herewith conclude that Food Research Lab provides new product development services.

Let’s create something Innovative and Delicious together
Food Research Lab strives for excellence in new Food, Beverage and Nutraceutical Product Research and Development by offering cutting edge scientific analysis and expertise.




