Nails
Our goal is to make your dream concept a commercial product, integrating our strong knowledge of ingredients and techniques to help you make the right decisions
Our goal is to make your dream concept a commercial product, integrating our strong knowledge of ingredients and techniques to help you make the right decisions
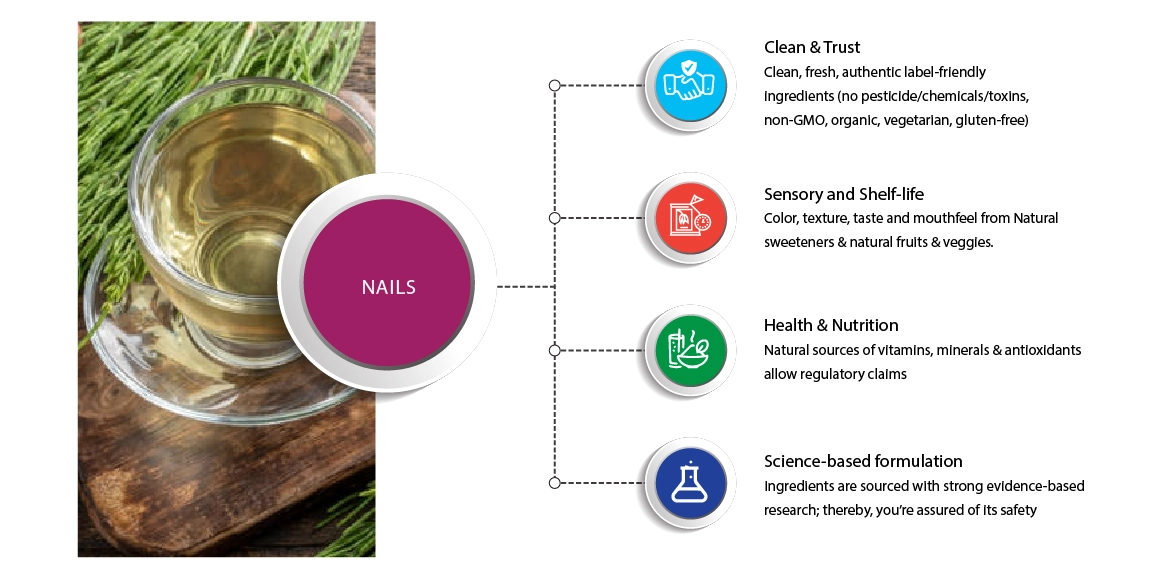
What aspects would you consider while developing a product that treats nails?
Co-creation from stakeholders and community input
Ingredient Focus
Clean Label
Sensory and Shelf Life
Regulatory: A prototype that meets the regulatory requirement governing food processing, including those established by the local and international regulations (e.g., FDA and EU regulations ) Science-Based Nutrition: An evidence-based prototype specifically built based on science to meet the targeted consumer's needs. In this case, the development of acne-reduction products demands oxidation-reduction. Evidence showed that dietary factors such as increased GI or GL, dairy products, and meat consumption in the diet were associated with acne. While probiotics and omega-3 fatty acids have been shown to decrease acne as it has the potential to decrease IGF-1 and inhibit pro-inflammatory leukotriene B4. Evidence showed that Brewer's yeast tablets, selenium, zinc, evening primrose oil, and vitamin E capsules are also helpful. Similarly, lycopene-rich ice cream was found to mitigate the damaging effects of oxidative stress. Keeping this, our formulation scientists develop products rich in antioxidants. We research the functional ingredients to achieve nutritargeting that has the capability to go through systemic circulation in active forms, distributed to all skin compartments, including sebum, subcutaneous fat, dermis, and epidermis, which is more efficient than applying cosmetics. We work along with manufacturers, dermatologists and physicians to test the scientific veracity of the claims.
Health Claim
1. Functional and Health claims as per the regulatory norms (e.g., no added artificial colours or flavours, and low in sugar)
We adopt a case-by-case approach to design nail curing (Nutricosmecutical) product development studies
We comply with Good Manufacturing Practices (GMP) guidelines/
Are FRL's Nail Formulations effective?
FRL's scientists and formulation experts identify new delivery mechanisms (such as liposomes) and research-active compounds to develop a highly functional nutraceutical product that provides the intended results. Through years of expertise and knowledge, we bring in the right solutions that fit your requirements.
At Food Research Lab, we give significance to the following aspects of developing a successful functional food product or a Nutracosmeceutical product. Any ingredient incorporated into the formulation needs to achieve its intended function in the human body at the same time must adhere to safety guidelines. At FRL, we substantiate this from many different types of scientific investigation, including secondary research for existing ingredients or, if it's novel, publishing the work in reputable, peer-reviewed journals. Data for this will also be derived from the purity and sustainability of the components (e.g., metal test), chemical comparisons and analysis, activities (e.g., ORAC or oxygen radical absorbance activities), testing in animal models, genomic/gene expression studies, in vitro bioassays and, most compelling of all, human clinical testing. Through years of expertise and knowledge, we bring in the right solutions that fit your requirements.
We follow quality measures to ensure the products are safe from both the supplier's and the buyer's perspectives. We carry out Marker Chemistry (e.g., HPLC, GC etc.), Microbiology tests (e.g., Pseudomonas, Salmonella, Escherichia Coli), heavy metal analysis (e.g., lead, arsenic, cadmium kept below the regulatory limits), Contamination (e.g., metal flakes from machinery), physical characteristics (e.g., powders for tablets must have low moisture content) and stability (e.g., the time for a product from initial production). We use compendium methods (USP and European Pharmacopeia) for an extensive validation process.
We follow a specific set of regulations that a product must adhere to. This includes manufacturing flow chart, solvents, quantitative ingredient lists, physical characteristics, GMO status, certifications, radiations, pesticide/chemical/ residue, contaminants, microbiology, allergens, animal derivation, and safety (Standard Material Safety Data Sheet – toxicity or clinical testing). We ensure to assist in vetting and clearing the ingredient supplier completes clearance through the target country's regulatory agency before its inclusion in formulas. Through our years of experience, our team can speed up the acceptance and inclusion of new material. Our product development team complies with the Food, Drug and Cosmetics Act (FDA 2009) and the Fair Packaging and Labeling Act (Federal Trade Commission 2011). Our team ensures to use of only acceptable ingredients that are found in the INCI, International Nomenclature of Cosmetic Ingredients, allocated by the American Cosmetic Association
At FRL's food research lab, we formulate food and nutraceuticals for specific products targeting acne. FRL's scientific expertise partners to create winning products that meet the above requirements.
For further information or prices please contact us: